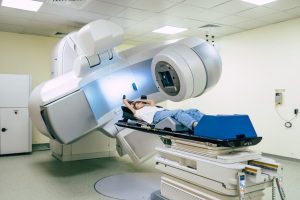
External vs. Internal Radiation for Breast Cancer
If you’ve been diagnosed with breast cancer, you may have heard radiation therapy discussed as part of your treatment plan. This approach is commonly used
HIPAA Alert: Potential Data Breach Learn More
Questions on Oncology, Hematology and/or Infusion Clinical Services due to COVID-19 Crisis – CALL 833-698-1623
Important Information for Our Patients Regarding the Coronavirus.
RCCA Providing Area Cancer Patients with Access to Care During Coronavirus Outbreak
RCCA Offering Patients Virtual Visits During Coronavirus Pandemic
Oncologists added more than a dozen new therapies to their cancer-fighting arsenal in 2025, and the year ahead holds the promise of further treatment advances, says Iuliana Shapira, MD.
“The Food and Drug Administration approved 13 novel cancer therapies last year as well as another agent that plays a key role in preparing patients with leukemia to undergo stem cell transplantation,” says Dr. Shapira. “Those 14 agents account for almost one-third of the novel therapies approved by the FDA in 20251,” adds Dr. Shapira, who serves as Chief Medical Officer of Regional Cancer Care Associates (RCCA), one of the nation’s largest networks of cancer specialists. The board-certified medical oncologist and hematologist explains that novel therapies are new drugs not previously approved or marketed for cancer treatment in the United States.

Seth H. Berk, MD, says, “The therapies approved in 2025 include five treatments for lung cancer. These treatments are particularly welcome because lung cancer continues to claim more lives each year than any other type of cancer.” Dr. Berk, a board-certified medical oncologist and hematologist who practices at RCCA’s Moorestown, NJ, office, adds that other newly approved agents include two therapies for breast cancer, one for ovarian cancer, one for brain cancer, one for acute myeloid leukemia, and one for multiple myeloma.
Dr. Berk continues, “At RCCA, our goal is to enable patients to receive cutting-edge therapies at community-based practices close to their homes. My colleagues and I at RCCA’s 22 locations throughout New Jersey, Connecticut, Massachusetts, and the Washington, D.C., area rapidly incorporate newly approved treatments into the care we provide. We also offer our patients the ability to participate in clinical trials of promising therapies in late stages of evaluation, so that they truly have access to the latest in cancer care.”
“Twelve of the novel therapies approved in 2025 are indicated for treatment of advanced disease, such as cases in which metastases have spread far from the cancer’s original site or when a patient experiences relapse after a favorable response to initial treatment. This preponderance of medications developed to treat later-stage cancer speaks to our increasing ability to offer patients effective interventions and hope even in situations where the focus is not on cure but rather on controlling cancer and prolonging life for an extended period while providing a good quality of life,” Dr. Berk notes.
Dr. Shapira adds that 10 of the newly approved agents are targeted therapies – medications that act against a specific genetic mutation or biological process driving a person’s cancer. “The last several years have seen a dramatic expansion in the number of targeted therapies available to oncologists. This enables us to practice precision medicine, tailoring our treatments to the underlying causes of a particular patient’s disease. The rise of targeted therapies is a major factor in the improved outcomes we have been able to achieve for our patients. And while these therapies are not without side effects, the focused nature of their activity means that they generally have less impact on healthy cells than other types of treatment, which can help us avoid or minimize many of the side effects seen with chemotherapy,” she says.
Dr. Berk adds that in addition to the novel cancer therapies approved in 2025, the FDA endorsed expanded uses, new formulations, or new delivery methods for another 40 already-approved cancer medications.2 In many of those cases, he explains, a drug that was indicated to treat one type of solid tumor or blood-based malignancy secured FDA approval for use in another form of cancer based on the results of additional clinical trials.
He says, “In summary, 2025 saw several major steps forward in our ability to treat a wide variety of cancers. We still have a long way to go as – tragically – the year also saw an estimated 618,000 people across the U.S. die from cancer,3 but the progress we’re making is very real, very significant, and will only gain speed in 2026.”
Dr. Shapira adds, “It is important to remember that every medication we are able to offer people with cancer today is available only because at some earlier point, other people facing the same disease stepped forward to participate in a clinical trial that demonstrated the efficacy and safety of that therapy. I have three wishes for people this year. First, that they will enjoy good health and will follow the lifestyle practices that reduce their risk of developing cancer. Second, that they will obtain all the indicated screenings that are critical to identifying cancer in its earliest stages. And, third, if – unfortunately – they should receive a diagnosis of cancer, they realize that they have abundant reason for hope and that, if they are candidates for a clinical trial, they consider participating in a study in order to receive the latest therapies under closely supervised conditions while advancing our ability to treat cancer effectively.”
Dr. Berk and Dr. Shapira are among 90+ medical oncologists and hematologists who practice with Regional Cancer Care Associates (RCCA), one of the nation’s largest networks of oncology specialists. RCCA has more than 20 locations near you across New Jersey, Connecticut, Massachusetts, and the Washington, D.C., area. RCCA’s cancer specialists see more than 30,000 new patients each year and provide care to more than 265,000 established patients, collaborating closely with those patients’ other physicians. RCCA physicians offer patients innovative therapies, including immunotherapies and targeted therapy, as well as access to approximately 300 clinical trials. In addition to serving patients who have solid tumors, blood-based cancers, and benign blood disorders, RCCA care centers also provide infusion services to people with a number of non-oncologic conditions—including multiple sclerosis, Crohn’s disease, asthma, iron-deficiency anemia, and rheumatoid arthritis—who take intravenously-administered medications.
To learn more about RCCA, call 844-346-7222 or contact RCCA.
References
Table: Novel cancer therapies receiving FDA approval in 20251
| Medication | Approval Date | Indication |
| Hyrnuo® (sevabertinib) | 11/19/2025 | To treat locally advanced or metastatic non-squamous non-small cell lung cancer with tumors that have activating HER2 tyrosine kinase domain activating mutations in patients who received a systemic therapy |
| Komzifti™ (ziftomenib) | 11/13/2025 | To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible nucleophosmin 1 mutation who have no satisfactory alternative treatment options |
| Inluriyo™ (imlunestrant) | 9/25/2025 | To treat estrogen receptor-positive, human epidermal growth factor receptor 2-negative, estrogen receptor-1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy |
| Keytruda Qlex™ (pembrolizumab and berahyaluronidase alfa-pmph ) | 9/19/2025 | To treat adult and pediatric (12 years and older) solid tumor indications approved for the intravenous formulation of pembrolizumab |
| Hernexeos® (zongertinib) | 8/8/2025 | To treat adults with unresectable or metastatic non-squamous non-small cell lung cancer whose tumors have HER2 tyrosine kinase domain activating mutations, as detected by an FDA-approved test, and who have received prior systemic therapy |
| Modeyso™ (dordaviprone) | 8/6/2025 | To treat diffuse midline glioma harboring an H3 K27M mutation with progressive disease following prior therapy |
| Zegfrovy® (sunvozertinib) | 7/2/2025 | To treat locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations, as detected by an FDA-approved test, with disease progression on or after platinum-based chemotherapy |
| Lynozyfic™ (linvoseltamab-gcpt) | 7/2/2025 | To treat relapsed or refractory multiple myeloma after at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti CD38 monoclonal antibody |
| Ibtrozi™ (taletrectinib) | 6/11/2025 | To treat locally advanced or metastatic ROS1-positive non-small cell lung cancer |
| Emrelis™ (telisotuzumab vedotin-tllv) | 5/14/2025 | To treat locally advanced or metastatic, non-squamous non-small cell lung cancer (NSCLC) with high c-Met protein overexpression after prior systemic therapy |
(avutometinib and defactinib) | 5/8/2025 | To treat KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) after prior systemic therapy |
| penpulimab-kcqx | 4/23/2025 | In combination with either cisplatin or carboplatin and gemcitabine, to treat adults with recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (NPC), or as a single agent while on or after platinum-based chemotherapy and at least one other prior line of therapy |
| Grafapex™ (treosulfan) | 1/21/2025 | For use in combination with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome |
| Datroway® (datopotamab deruxtecan-dlnk) | 1/17/2025 | To treat unresectable or metastatic, HR-positive, HER2-negative breast cancer in patients who have received prior endocrine-based therapy and chemotherapy for unresectable or metastatic disease |
Source: U.S. Food and Drug Administration (FDA). Novel drug approvals for 2025. Available at https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2025. Accessed December 31, 2025.

“At RCCA, our goal is to enable patients to receive cutting-edge therapies at community-based practices close to their homes. My colleagues and I rapidly incorporate newly approved treatments into the care we provide.”
- Seth H. Berk, MD

“The last several years have seen a dramatic expansion in the number of targeted therapies available to oncologists. This enables us to practice precision medicine, tailoring our treatments to the underlying causes of a particular patient’s disease.”
- Iuliana Shapira, MD
For more information or to schedule an appointment,
call 844-346-7222. You can also schedule an appointment by calling the RCCA location nearest you.

If you’ve been diagnosed with breast cancer, you may have heard radiation therapy discussed as part of your treatment plan. This approach is commonly used

Cancer and its treatment often impact a person’s quality of life. In the case of prostate cancer, a man’s sex life may be affected. Sexual

Lung cancer is commonly considered a smoker’s disease, as cigarette smoking is the most significant risk factor for its development. However, not all cases are

Regional Cancer Care Associates is one of fewer than 200 medical practices in the country selected to participate in the Oncology Care Model (OCM); a recent Medicare initiative aimed at improving care coordination and access to and quality of care for Medicare beneficiaries undergoing chemotherapy treatment.