
Does Smoking Marijuana Cause Lung Cancer?
Marijuana is a drug that can cause a euphoric, relaxing feeling. Research indicates that it also can play a role in pain relief and help
HIPAA Alert: Potential Data Breach Learn More
Questions on Oncology, Hematology and/or Infusion Clinical Services due to COVID-19 Crisis – CALL 833-698-1623
Important Information for Our Patients Regarding the Coronavirus.
RCCA Providing Area Cancer Patients with Access to Care During Coronavirus Outbreak
RCCA Offering Patients Virtual Visits During Coronavirus Pandemic
Although multiple myeloma is an incurable cancer, doctors can prescribe various treatments to help patients manage their symptoms and slow the disease’s progress. The experienced oncologists and hematologists of Regional Cancer Care Associates treat numerous cancer types and blood disorders, including multiple myeloma. Individuals throughout New Jersey, Connecticut, and Maryland, as well as the Washington, D.C., area, rely on Regional Cancer Care Associates for compassionate care and cutting-edge treatment.
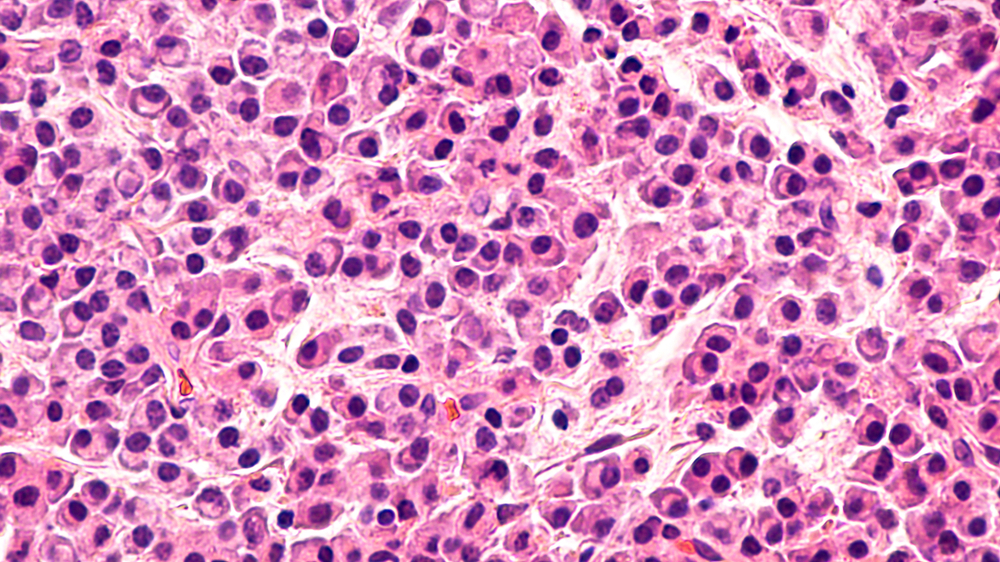
Diagnosing a patient with multiple myeloma can be challenging because symptoms often do not show until the disease has progressed significantly. Other times, signs of this cancer may seem related to a different condition, or the patient has no symptoms at all. The following are some indications a person may have multiple myeloma:
The oncologists and hematologists of Regional Cancer Care Associates employ a variety of means to diagnose multiple myeloma and assigning it a stage that reflects the nature and extent of disease. Blood tests are used to assess levels of albumin, beta-2 microglobulin, calcium, uric acid, and lactate dehydrogenase, and – potentially – other biomarkers. Urine tests and examination of a bone marrow sample also can be valuable, as can imaging studies, such as X-ray, magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET).
Staging enables doctors to characterize the disease’s nature and extent, and to make treatment decisions accordingly.
The five-year survival rate for people with multiple myeloma ranges widely, from 40% to 82%, depending on the nature and extent of the disease. As with other cancers, the earlier multiple myeloma is detected, the sooner the patients can begin treatment, and – in general – the longer their life expectancy. Although the disease cannot be cured at this time, treatment also can prevent, relieve, or minimize symptoms. Treatment for multiple myeloma includes:

Besides medications and therapies, physicians may recommend lifestyle changes to mitigate symptoms and impede cancer’s progression. Some behaviors patients with multiple myeloma may consider implementing include:
Multiple myeloma — and cancer in general — takes a tremendous toll on a person’s life, both physically and emotionally. However, receiving the latest treatments from compassionate and experienced providers can provide patients not only with the best possible outcomes but also with patients’ peace of mind. At Regional Cancer Care Associates, oncologists and hematologists provide patients with cutting-edge diagnoses and treatments for cancer and blood disorders. Contact one of the 25 locations in New Jersey, Connecticut, Maryland, and the Washington, D.C., area to schedule an appointment.
At Regional Cancer Care Associates (RCCA), we take a comprehensive, “whole-life” approach, treating you as a whole person — not just your disease. We treat your cancer in an individualized and medically advanced manner. At RCCA, you’ll be diagnosed, treated and cared for by nationally-recognized experts who are close to your home.
You can schedule an appointment or get more information by calling (844) 346-7222. You can also schedule an appointment by calling the RCCA location near you.

Marijuana is a drug that can cause a euphoric, relaxing feeling. Research indicates that it also can play a role in pain relief and help

If you’ve been diagnosed with prostate cancer, you’ll want to understand all your options and choose the approach that offers the best outcomes. For many

Chemotherapy is an effective cancer treatment, but it also causes side effects and a weakened immune system in many patients. Fortunately, there are ways patients
When standard cancer treatments aren’t providing the results you want, clinical trials may offer hope. Our physicians use clinical trials to study new treatments, helping transform cancer care for the better. You can enroll in a clinical trial to try groundbreaking treatment plans at zero cost to you.

Regional Cancer Care Associates is one of fewer than 200 medical practices in the country selected to participate in the Oncology Care Model (OCM); a recent Medicare initiative aimed at improving care coordination and access to and quality of care for Medicare beneficiaries undergoing chemotherapy treatment.